

The water is then injected onto a combustion column packed with platinum-coated alumina beads held at 680☌.

How to calculate ppm using glass standard free#
Ppm (parts per million) to % (parts per hundred)ĭivide the ppm amount by 1,000,000 and multiply by 100 to get %.ġ0,000 ppm = 10000/1,000,000 = 0.01 = 1.0%Ģ0,000 ppm = 20000/1,000,000 = 0.02 = 2.0%ĭivide the % value by 100 and multiply by 1,000,000 to get ppm.An acidified seawater sample (< pH 3) is sparged with CO 2 free air to remove inorganic carbon. Hence Molarity = 400ppm divided by 40,000mg/mol = 0.01M Using the mg/l concentration, the 40g Ca must be converted to milligrams by multiplying by 1000 to give 40,000mg. Or, Divide 400 mg by 1000 to get g/l = 0.4 g/l What is the Molarity of 400ppm Ca ions in an aqueous CaCO3 solution? Go here for ISE molarity/ppm conversions shown in Table III.Ĭonvert ppm to gram based or milligram based concentration.Į.g. Fluoride has a FW of 19, hence a 10 -3M concentration is equal to 19ppm, 1M is equal to 19,000 ppm and 1ppm is equal to 5.2 x 10 -5M. The FW of an ion species is equal to its concentration in ppm at 10 -3M. Molarity(M) x Atomic mass(At Wt) = grams per liter(g/l) What is the ppm concentration of calcium ion in 0.01M CaCO 3? pipette 10 ml into a 100 ml flask and make up to mark to give a final 10 -5 M solution.Ĭonvert molar concentration to grams per liter (Molarity x Atomic mass of solute), then convert to milligrams per liter (ppm) by multiplying by 1000.Į.g. into another 100 ml flask and make up to mark to give a 10 -4 M soln.Īnd from this 10 -4 M soln. into yet another 100 ml flask and make up to mark to give a 10 -3 M soln. Pipette again, 10 ml of this 10 -2 M soln. into another 100 ml flask and make up to the mark to give a 10 -2 M soln. Pipette 10 ml of the 1M stock into a 100 ml volumetric flask and make up to the mark to give a 10 -1 M soln. Making up 10 -1 M to 10 -5 M solutions from a 1M stock solution. 12.5 mls of 100 ppm in 50 ml volume will give a 25 ppm solution = no of mls for req volĮxample : Make up 50 mls vol of 25 ppm from 100 ppm standard.Ģ5 x 50 / 100 = 12.5 mls.
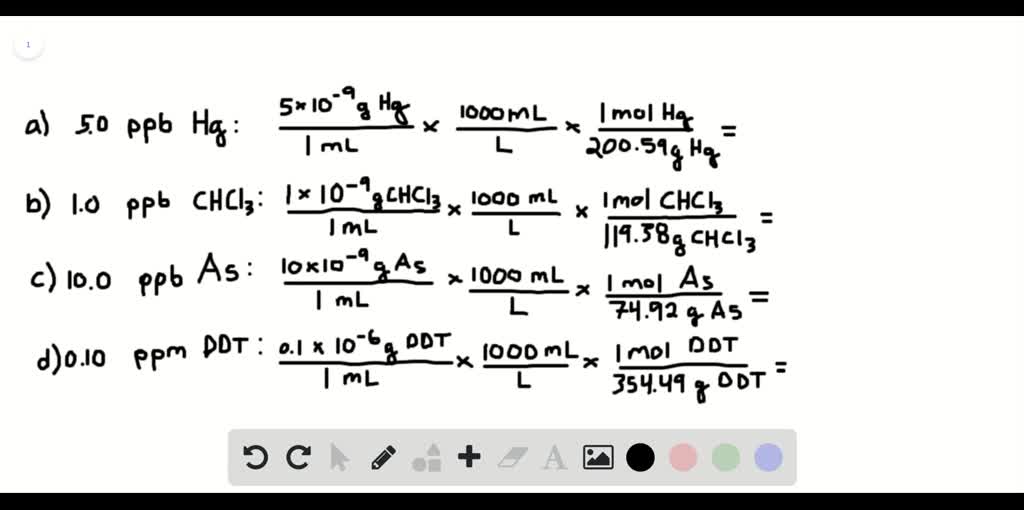
The Formula below can be used to calculate the the V1 component only. This means that 66.7 mls of the 6.00ppm solution diluted to a final volume of 4 liters will give a concentration of 0.100 ppm. This equation applies to all dilution problems.Ĭ1 (initial conc) x V1 (initial volume) = C2 (final conc) x V2 (final volume)Įxample : What volume of 6.00 ppm solution must be used to give 4.00 liters of a 0.100 ppm solution? Hence, weigh out 1.432g KH 2PO 4 and dissolve in 1 liter volume to make a 1000 ppm PO 4 standard.Ĭlick this link for Atomic absorption standards Make a 1000 ppm phosphate standard using the salt KH 2PO 4ġg PO 4 in relation to FW of salt = 136.09 / 95 = 1.432g. Hence, weigh out 2.542g NaCl and dissolve in 1 liter volume to make a 1000 ppm Na standard.Į.g. Make a 1000 ppm standard of Na using the salt NaCl.ġg Na in relation to FW of salt = 58.44 / 23 = 2.542g. nitric or hydrochloric acid, and make up to the mark in 1 liter volume deionised water.Į.g. From the pure metal : weigh out accurately 1.000g of metal, dissolve in 1 : 1 conc. PPB = Parts per billion = ug/L = ng/g = ng/ml = pg/mg = 10 -9ġ. Ppm = ug/g =ug/ml = ng/mg = pg/ug = 10 -6ġ gram pure element disolved in 1000ml = 1000 ppm PPM is derived from the fact that the density of water is taken as 1kg/L = 1,000,000 mg/L, and 1mg/L is 1mg/1,000,000mg or one part in one million. One thousanth of a gram is one milligram and 1000 ml is one liter, so that 1 ppm = 1 mg per liter = mg/Liter. One gram in 1000 ml is 1000 ppm and one thousandth of a gram (0.001g) in 1000 ml is one ppm. PPM is a term used in chemistry to denote a very, very low concentration of a solution.
How to calculate ppm using glass standard serial#
PPM conversion values and serial dilutions :


 0 kommentar(er)
0 kommentar(er)
